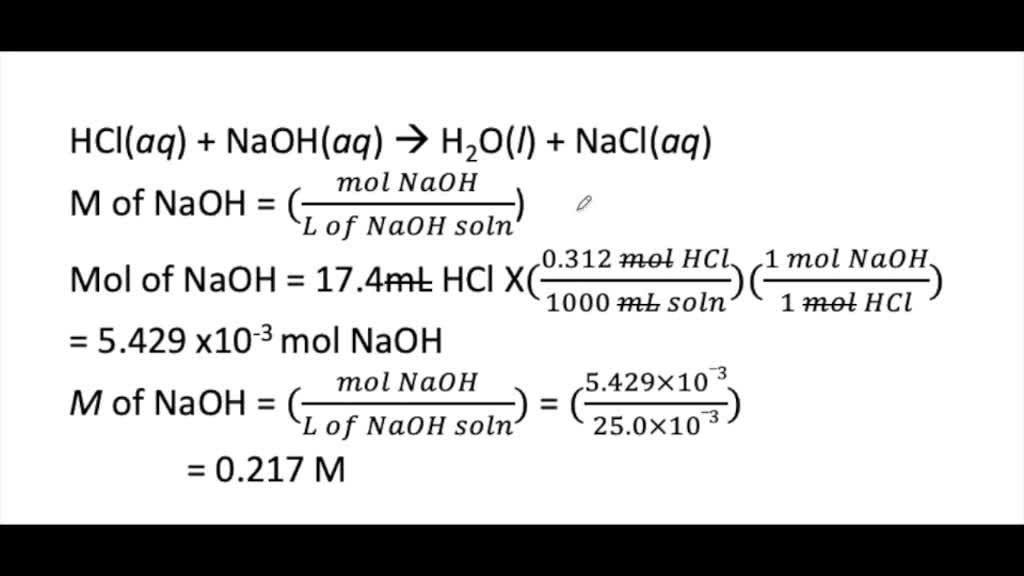How To Calculate Concentration In Mol Dm-3 In Titration . Distilled water can be added to the. In this, and the rest of these calculations, i am. Naoh + hcl nacl + h 2 o. the concentration of a solution can be calculated using: add solution from burette whilst swirling the mixture and add dropwise at end point. the first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. revision notes on using concentrations in mol/dm3 for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. In each case, you start with 25 cm 3 of one of the solutions. In our example we would accurately.
from www.numerade.com
Distilled water can be added to the. In our example we would accurately. Naoh + hcl nacl + h 2 o. the concentration of a solution can be calculated using: In each case, you start with 25 cm 3 of one of the solutions. the first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. In this, and the rest of these calculations, i am. add solution from burette whilst swirling the mixture and add dropwise at end point. revision notes on using concentrations in mol/dm3 for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams.
SOLVEDCalculate the concentration (in molarity) of an mathrm{NaOH
How To Calculate Concentration In Mol Dm-3 In Titration revision notes on using concentrations in mol/dm3 for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. add solution from burette whilst swirling the mixture and add dropwise at end point. Distilled water can be added to the. In our example we would accurately. In each case, you start with 25 cm 3 of one of the solutions. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Naoh + hcl nacl + h 2 o. the first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. In this, and the rest of these calculations, i am. revision notes on using concentrations in mol/dm3 for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. the concentration of a solution can be calculated using:
From quizdbcarronades.z21.web.core.windows.net
How To Calculate Final Molar Concentration How To Calculate Concentration In Mol Dm-3 In Titration In this, and the rest of these calculations, i am. revision notes on using concentrations in mol/dm3 for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. Distilled water can be added to the. In each case, you start with 25 cm 3 of one of the solutions. the first step is to. How To Calculate Concentration In Mol Dm-3 In Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate Concentration In Mol Dm-3 In Titration Distilled water can be added to the. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. add solution from burette whilst swirling the mixture and add dropwise at end point. Naoh + hcl nacl + h 2 o. In our example we would accurately. revision notes on using concentrations in mol/dm3 for. How To Calculate Concentration In Mol Dm-3 In Titration.
From www.slideserve.com
PPT Titration calculations PowerPoint Presentation, free download How To Calculate Concentration In Mol Dm-3 In Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. In each case, you start with 25 cm 3 of one of the solutions. the first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. Distilled water can be added to. How To Calculate Concentration In Mol Dm-3 In Titration.
From slideplayer.com
Titration Calculation ppt download How To Calculate Concentration In Mol Dm-3 In Titration In our example we would accurately. the concentration of a solution can be calculated using: add solution from burette whilst swirling the mixture and add dropwise at end point. the first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. In each case, you. How To Calculate Concentration In Mol Dm-3 In Titration.
From www.savemyexams.co.uk
Using Concentrations in mol/dm3 (3.4.1) AQA GCSE Chemistry Revision How To Calculate Concentration In Mol Dm-3 In Titration Naoh + hcl nacl + h 2 o. add solution from burette whilst swirling the mixture and add dropwise at end point. revision notes on using concentrations in mol/dm3 for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. In each case, you start with 25 cm 3 of one of the solutions.. How To Calculate Concentration In Mol Dm-3 In Titration.
From www.slideserve.com
PPT Concentration PowerPoint Presentation, free download ID3874312 How To Calculate Concentration In Mol Dm-3 In Titration Naoh + hcl nacl + h 2 o. the first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. add solution from burette whilst swirling the mixture and add dropwise at end point. the concentration of a solution can be calculated using: In a. How To Calculate Concentration In Mol Dm-3 In Titration.
From www.numerade.com
SOLVED The relationship between concentration, amount of material How To Calculate Concentration In Mol Dm-3 In Titration add solution from burette whilst swirling the mixture and add dropwise at end point. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. the concentration of a solution can be calculated using: Naoh + hcl nacl + h 2 o. revision notes on using concentrations in mol/dm3 for the aqa gcse. How To Calculate Concentration In Mol Dm-3 In Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate Concentration In Mol Dm-3 In Titration Naoh + hcl nacl + h 2 o. In this, and the rest of these calculations, i am. In our example we would accurately. the concentration of a solution can be calculated using: the first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. . How To Calculate Concentration In Mol Dm-3 In Titration.
From www.savemyexams.co.uk
Using Concentrations in mol/dm3 (3.4.1) AQA GCSE Chemistry Revision How To Calculate Concentration In Mol Dm-3 In Titration Distilled water can be added to the. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. add solution from burette whilst swirling the mixture and add dropwise at end point. the first step is to measure out a known volume of the sample that you want to find the concentration of into. How To Calculate Concentration In Mol Dm-3 In Titration.
From exommzgkk.blob.core.windows.net
Titration Concentration Of Solution at Tony Pelletier blog How To Calculate Concentration In Mol Dm-3 In Titration In our example we would accurately. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. In each case, you start with 25 cm 3 of one of the solutions. In this, and the rest of these calculations, i am. add solution from burette whilst swirling the mixture and add dropwise at end point.. How To Calculate Concentration In Mol Dm-3 In Titration.
From lessonlibraryhauliers.z14.web.core.windows.net
How To Calculate Molarity With Examples How To Calculate Concentration In Mol Dm-3 In Titration the first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. the concentration of a solution can be calculated using: In our example we would accurately. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. In this, and the. How To Calculate Concentration In Mol Dm-3 In Titration.
From www.chegg.com
Solved Calculate molar concentration ( mol*dm3 ) of How To Calculate Concentration In Mol Dm-3 In Titration revision notes on using concentrations in mol/dm3 for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. add solution from burette whilst swirling the mixture and add dropwise at end point. In this, and the rest of these calculations, i am. In each case, you start with 25 cm 3 of one of. How To Calculate Concentration In Mol Dm-3 In Titration.
From spmchemistry.blog.onlinetuition.com.my
Solute, Solvent and Solution SPM Chemistry How To Calculate Concentration In Mol Dm-3 In Titration In each case, you start with 25 cm 3 of one of the solutions. add solution from burette whilst swirling the mixture and add dropwise at end point. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. In our example we would accurately. Distilled water can be added to the. the first. How To Calculate Concentration In Mol Dm-3 In Titration.
From slideplayer.com
Titration Calculation ppt download How To Calculate Concentration In Mol Dm-3 In Titration the first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. add solution from burette whilst swirling the mixture and add dropwise at end point. the concentration of a solution can be calculated using: Distilled water can be added to the. revision notes. How To Calculate Concentration In Mol Dm-3 In Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate Concentration In Mol Dm-3 In Titration In our example we would accurately. Distilled water can be added to the. the first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. revision notes on using concentrations in mol/dm3 for the aqa gcse chemistry syllabus, written by the chemistry experts at save my. How To Calculate Concentration In Mol Dm-3 In Titration.
From www.numerade.com
SOLVED If 25,0 cm^3 of the NH3 is titrated with the HCL, calculate the How To Calculate Concentration In Mol Dm-3 In Titration In each case, you start with 25 cm 3 of one of the solutions. In our example we would accurately. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Distilled water can be added to the. In this, and the rest of these calculations, i am. the first step is to measure out. How To Calculate Concentration In Mol Dm-3 In Titration.
From www.slideserve.com
PPT Mole Calculations PowerPoint Presentation ID902714 How To Calculate Concentration In Mol Dm-3 In Titration Distilled water can be added to the. Naoh + hcl nacl + h 2 o. revision notes on using concentrations in mol/dm3 for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. the first step is to measure out a known volume of the sample that you want to find the concentration of. How To Calculate Concentration In Mol Dm-3 In Titration.
From www.researchgate.net
The concentration (mol/dm 3 ) of iron (CFe) and nickel (C Ni How To Calculate Concentration In Mol Dm-3 In Titration Distilled water can be added to the. revision notes on using concentrations in mol/dm3 for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. In our example we would accurately. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. In this, and the rest of these calculations, i. How To Calculate Concentration In Mol Dm-3 In Titration.